Abstract
Background: Mutations in the key tumor suppressor gene TP53 lead to inactivation of the protein p53, which leads to genome instability, oncogenesis, treatment resistance and an overall unfavorable prognosis in various malignancies. The specific TP53 Y220C variant has been described as one of the most frequent "hot spot" mutations associated with solid cancers. Recently, a Phase 1 clinical trial studying the novel Y220C-targeted small molecule PC14586, has shown preliminary safety and efficacy in patients with heavily treated solid tumors. No data has been reported about TP53 Y220C in hematologic malignancies to date. The aim of the present analysis is to evaluate the clinicopathologic characteristics of TP53 Y220C in hematologic malignancies.
Methods: We searched our molecular laboratory database for hematologic neoplasms with TP53 Y220C mutations and summarized the patient characteristics, disease features and outcomes for patients treated within the Department of Leukemia at MD Anderson. Overall survival (OS) was assessed by the Kaplan-Meier method calculated from the date of TP53 Y220C detection to the date of death from any cause or date of last follow-up.
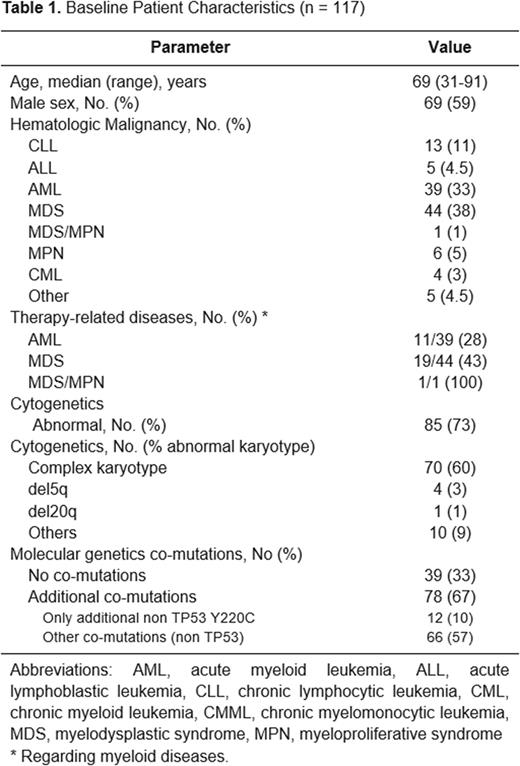
Results: We identified 126 patients with hematologic malignancies harboring a TP53 Y220C mutation. Nine patients with lymphoma or myeloma were treated outside our Department and were excluded from subsequent analysis. 117 patients had a diagnosis of acute or chronic leukemia or myelodysplastic syndrome (MDS) [Table 1]. Median age was 69 years (31-91) and 59% of patients were male. Acute myeloid leukemia (AML) and MDS accounted for 33% and 38%, respectively. Consistent with the increased incidence in TP53-mutated myeloid disorders, 28% of AML and 43% of MDS cases were therapy-related. Cytogenetics was abnormal in 85 patients (73%), with a complex karyotype occurring in 70 patients (60%). Among 73 patients with detailed VAF data, the TP53 Y220C mutation had a median VAF of 35% (1-96%). Seventy-eight patients (67%) had additional co-mutations; 12 patients (10%) had only additional TP53 mutations and the remaining 66 patients (57%) had additional variants involving genes other than TP53 (56 patients with known somatic mutations and 10 patients with variants of uncertain significance -VUS). With a median follow-up of 49 months, the median OS of patients with TP53 Y220C mutations was 10 months, recognizing that the treatments received were variable based on diagnosis, age, and comorbidities among other variables. Median OS was 6.7, 9.3 and 60 months for AML, MDS and CLL patients, respectively. Additional outcomes data will be forthcoming.
Conclusions: TP53 Y220C mutation is present in hematologic diseases, primarily in myeloid neoplasms (MDS and AML) and with higher frequency in therapy-related disease. Targeted therapies for this specific TP53 mutation would be of interest in this difficult to treat patient population.
Disclosures
Sasaki:Pfizer: Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Otsuka Pharmaceuticals: Honoraria; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Loghavi:PeerView: Honoraria; Amgen: Research Funding; Astellas: Research Funding; QualWorld: Consultancy; Abbvie: Consultancy, Current equity holder in publicly-traded company; GLG: Consultancy. Short:AstraZeneca: Consultancy; Pfizer: Consultancy; Stemline Therapeutics: Research Funding; Astellas: Research Funding; Takeda Oncology: Consultancy, Research Funding; Amgen: Consultancy, Honoraria; Novartis: Consultancy. Daver:Agios, Celgene, SOBI and STAR Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos and Jazz Pharmaceuticals: Other: Data monitoring committee member; Karyopham Therapeutics and Newave Pharmaceutical: Research Funding; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Novartis, Jazz, Amgen, Servier, Karyopharm, Trovagene, Trillium, Syndax, Gilead, Pfizer, Bristol Myers Squibb, Kite, Actinium, Arog, Immunogen, Arcellx, and Shattuck: Consultancy, Other: Advisory Role; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Gilead, Immunogen, Pfizer, Bristol Myers Squibb, Trovagene, Servier, Novimmune, Incyte, Hanmi, Fate, Amgen, Kite, Novartis, Astex, KAHR, Shattuck, Sobi, Glycomimetics, Trillium: Research Funding. Borthakur:Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy; Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding; Pacylex, Novartis, Cytomx, Bio Ascend: Membership on an entity's Board of Directors or advisory committees. Ravandi:Amgen: Honoraria, Research Funding; AstraZeneca: Consultancy; Astellas: Consultancy, Honoraria, Research Funding; Syos: Consultancy, Honoraria, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Novartis: Consultancy; Abbvie: Consultancy, Honoraria, Research Funding; Xencor: Research Funding; Amgen: Honoraria, Research Funding; Biomea Fusion, Inc.: Research Funding; Prelude: Research Funding; Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding. Kantarjian:KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Research Funding; ImmunoGen: Research Funding; Jazz Pharmaceuticals: Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; NOVA Research: Honoraria; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Research Funding; Takeda: Honoraria. Andreeff:Aptose: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Research Funding; Reata: Current holder of stock options in a privately-held company; Brooklyn ITX: Research Funding; German Research Council: Membership on an entity's Board of Directors or advisory committees; Medicxi: Consultancy; Glycomimetics: Consultancy; Syndax: Consultancy, Research Funding; Pinot Bio: Research Funding; CLL Foundation: Membership on an entity's Board of Directors or advisory committees; Senti Bio: Consultancy, Research Funding; Chimerix: Current holder of stock options in a privately-held company; Oncolyze: Current holder of stock options in a privately-held company; NCI: Membership on an entity's Board of Directors or advisory committees; Oxford Biomedical UK: Research Funding; Leukemia & Lymphoma Society: Membership on an entity's Board of Directors or advisory committees; Cancer UK: Membership on an entity's Board of Directors or advisory committees; Breast Cancer Research Foundation: Research Funding; Daiichi-Sankyo Inc.: Consultancy, Research Funding; Kintor Pharmaceutical: Research Funding. DiNardo:Cleave: Research Funding; ImmuneOnc: Honoraria, Research Funding; Bluebird Bio: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Gilead: Honoraria; Astex: Research Funding; AbbVie: Consultancy, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Foghorn: Honoraria, Research Funding; Novartis: Honoraria; Astellas: Honoraria; Servier: Consultancy, Honoraria, Research Funding; Jazz: Honoraria; Forma: Research Funding; Kura: Honoraria, Membership on an entity's Board of Directors or advisory committees; LOXO: Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.